Textbook Question
Write the formula for the conjugate acid for each of the following bases:
a. CO32-
846
views

 Verified step by step guidance
Verified step by step guidance


Write the formula for the conjugate acid for each of the following bases:
a. CO32-
Write the formula for the conjugate acid for each of the following bases:
c. H2PO4-
Identify the Brønsted–Lowry acid–base pairs in each of the following equations:
b. NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
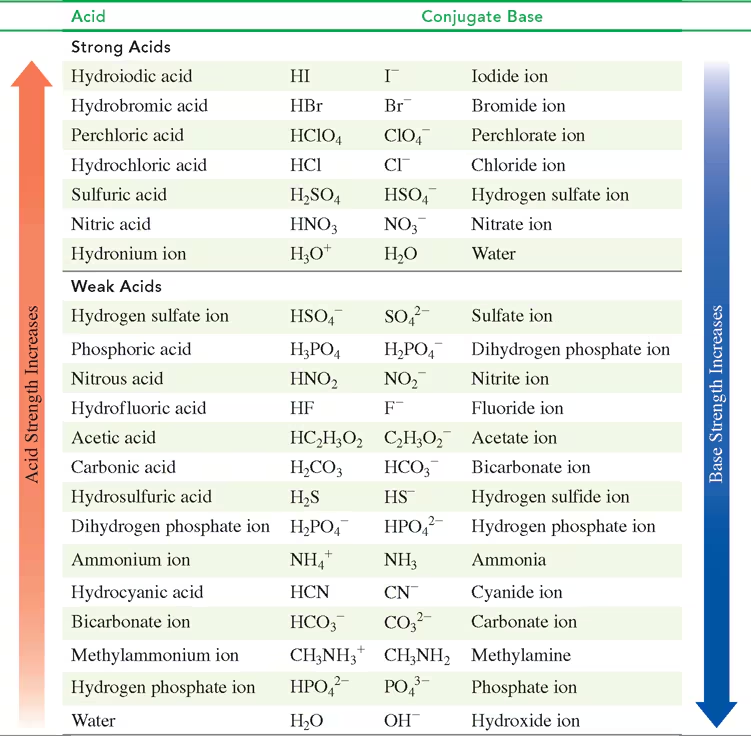
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
b. H3PO4 or HSO4-
What is meant by the term reversible reaction?
In an acidic solution, how does the concentration of H3O+ compare to the concentration of OH-?