Textbook Question
Give the name for the product from the hydrogenation of each of the following:
a. 3-methyl-2-pentene
867
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 66b
Problem 66b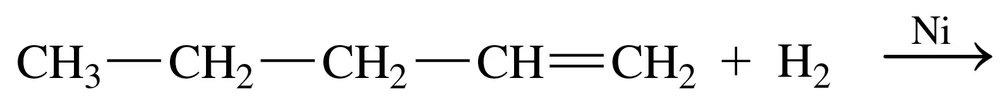
 Verified step by step guidance
Verified step by step guidance


Give the name for the product from the hydrogenation of each of the following:
a. 3-methyl-2-pentene
Give the name for the product from the hydrogenation of each of the following:
c. cyclopropene
Draw the condensed structural or line-angle formula for the product of each of the following:
a.
Draw the condensed structural formulas for all the possible alkane isomers that have a total of six carbon atoms and a four-carbon chain.
Draw the condensed structural formulas for all the possible haloalkane isomers that have four carbon atoms and a bromine.
Consider the compound ethylcyclopentane.
a. Draw the line-angle formula for ethylcyclopentane.