Textbook Question
Write the balanced chemical equation for the complete combustion of each of the following compounds:
b. cyclopropane
892
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 24d
Problem 24d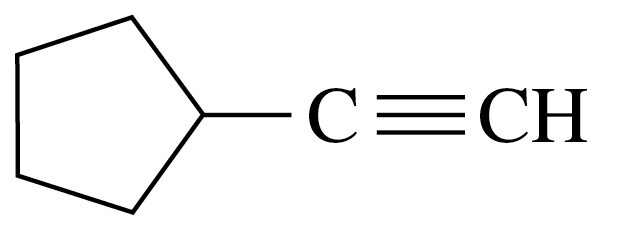
 Verified step by step guidance
Verified step by step guidance


Write the balanced chemical equation for the complete combustion of each of the following compounds:
b. cyclopropane
Write the balanced chemical equation for the complete combustion of each of the following compounds:
c. 2,3-dimethylhexane
Identify the following as alkanes, alkenes, cycloalkenes, or alkynes:
a.
Give the IUPAC name for each of the following:
c.
Give the IUPAC name for each of the following:
d.
Give the IUPAC name for each of the following:
b.