Textbook Question
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
c.
572
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 54b
Problem 54b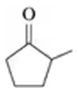
 Verified step by step guidance
Verified step by step guidance
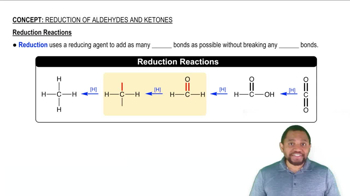
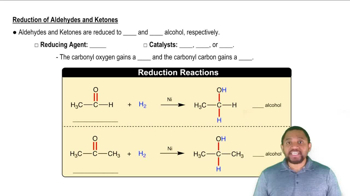

Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
c.
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
b.
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
a.
Give the IUPAC name for each of the following:
a.
Give the IUPAC name for each of the following:
b.
Give the IUPAC name for each of the following:
c.