Which of the following solvents might be used to dissolve an oil stain?
a. water
b. CCl4
c. diethyl ether
d. benzene
e. NaCl solution

 Verified step by step guidance
Verified step by step guidance

Which of the following solvents might be used to dissolve an oil stain?
a. water
b. CCl4
c. diethyl ether
d. benzene
e. NaCl solution
What are some functions of lipids in the body?
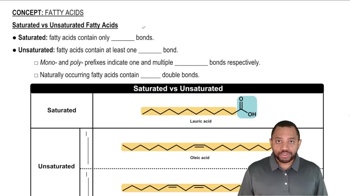
Describe some similarities and differences in the structures of a saturated fatty acid and an unsaturated fatty acid.
Draw the line-angle formula for each of the following fatty acids:
a. palmitic acid
For each of the following fatty acids, give the shorthand notation for the number of carbon atoms and double bonds, and classify as saturated, monounsaturated, or polyunsaturated:
a. lauric acid
For each of the following fatty acids, give the shorthand notation for the number of carbon atoms and double bonds, and classify as saturated, monounsaturated, or polyunsaturated:
a. linoleic acid