Identify the amino acids and type of interaction that occurs between the following R groups in tertiary protein structures:
a.

 Verified step by step guidance
Verified step by step guidance


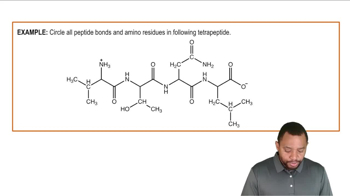
Identify the amino acids and type of interaction that occurs between the following R groups in tertiary protein structures:
a.
Identify the amino acids and type of interaction that occurs between the following R groups in tertiary protein structures:
c. —CH2—SH and HS—CH2—
What type of interaction would you expect between the following in a tertiary structure?
a. threonine and glutamine
Would you expect to find this segment at the center or at the surface of a protein? Why?
Seeds and vegetables are often deficient in one or more essential amino acids. Using the following table, state whether each combination provides all of the essential amino acids:
b. lima beans and cornmeal
Seeds and vegetables are often deficient in one or more essential amino acids. Using the table in problem 16.63, state whether each combination provides all of the essential amino acids.
<IMAGE>
a. rice and lima beans