Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
a. The inhibitor has a structure similar to the substrate.

 Verified step by step guidance
Verified step by step guidance

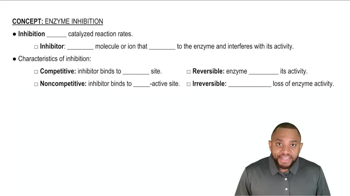
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
a. The inhibitor has a structure similar to the substrate.
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
d. The structure of the inhibitor is not similar to the substrate.
Oxaloacetate is an inhibitor of succinate dehydrogenase.
a. Would you expect oxaloacetate to be a competitive or a noncompetitive inhibitor? Why?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
a. Does the antibiotic inhibit enzymes in humans?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
c. Is amoxicillin a reversible or irreversible inhibitor?
Ethylene glycol (HO—CH2—CH2—OH) is a major component of antifreeze. If ingested, it is first converted to HOOC—CHO (oxoethanoic acid) and then to HOOC—COOH (oxalic acid), which is toxic.
<IMAGE>
a. What class of enzyme catalyzes the reactions described?