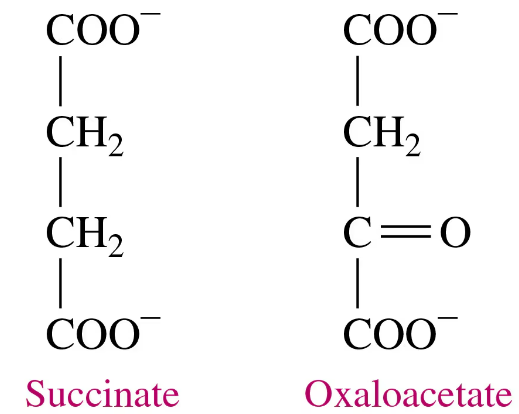
Refer to the graph in problem 16.45 to determine if the reaction rate in each condition will be at the optimum rate or not.
<IMAGE>
a. trypsin, pH 5.0

 Verified step by step guidance
Verified step by step guidance


Refer to the graph in problem 16.45 to determine if the reaction rate in each condition will be at the optimum rate or not.
<IMAGE>
a. trypsin, pH 5.0
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
a. The inhibitor has a structure similar to the substrate.
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
d. The structure of the inhibitor is not similar to the substrate.
Methanol and ethanol are oxidized by alcohol dehydrogenase. In methanol poisoning, ethanol is given intravenously to prevent the formation of formaldehyde that has toxic effects.
b. Would ethanol compete for the active site or bind to a different site?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
a. Does the antibiotic inhibit enzymes in humans?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
c. Is amoxicillin a reversible or irreversible inhibitor?