Trypsin, a peptidase that hydrolyzes polypeptides, functions in the small intestine at an optimum pH of 7.7 to 8.0. How is the rate of a trypsin-catalyzed reaction affected by each of the following conditions?
b. running the reaction at 75 °C

 Verified step by step guidance
Verified step by step guidance


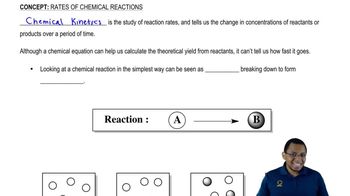
Trypsin, a peptidase that hydrolyzes polypeptides, functions in the small intestine at an optimum pH of 7.7 to 8.0. How is the rate of a trypsin-catalyzed reaction affected by each of the following conditions?
b. running the reaction at 75 °C
Pepsin, a peptidase that hydrolyzes proteins, functions in the stomach at an optimum pH of 1.5 to 2.0. How is the rate of a pepsin-catalyzed reaction affected by each of the following conditions?
a. changing the pH to 5.0
The following graph shows the activity versus pH curves for pepsin, sucrase, and trypsin. Estimate the optimum pH for each.
<IMAGE>
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
a. The inhibitor has a structure similar to the substrate.
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
d. The structure of the inhibitor is not similar to the substrate.
Oxaloacetate is an inhibitor of succinate dehydrogenase.
a. Would you expect oxaloacetate to be a competitive or a noncompetitive inhibitor? Why?