Textbook Question
Draw the condensed structural formula for CMP.
752
views

 Verified step by step guidance
Verified step by step guidance

Draw the condensed structural formula for CMP.
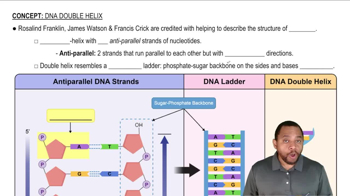
What is similar about the primary structure of RNA and DNA?
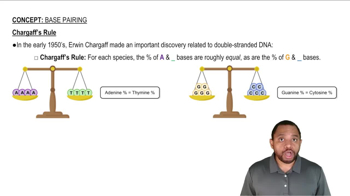
If the DNA double helix in salmon contains 28% adenine, what is the percentage of thymine, guanine, and cytosine?
Write the complementary base sequence for each of the following DNA segments:
c. G G C C T A C C T T A A C G A C G
Match the following statements with rRNA, mRNA, or tRNA:
c. carries genetic information from the nucleus to the ribosomes
Match the following statements with rRNA, mRNA, or tRNA:
a. combines with proteins to form ribosomes