Textbook Question
What is meant by the rate of a reaction?
2324
views

 Verified step by step guidance
Verified step by step guidance



What is meant by the rate of a reaction?
How would each of the following change the rate of the reaction shown here?
2 SO2(g) + O2(g) → 2 SO3(g)
a. adding some SO2(g)
How would each of the following change the rate of the reaction shown here?
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
c. removing some H2(g)
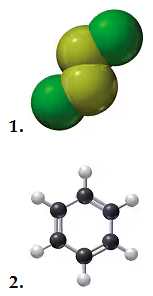
Using the models of the molecules (black = C, white = H, yellow = S, red = O), determine each of the following for models of compounds 1 and 2:
c. number of moles in 10.0 g
Ibuprofen, an anti-inflammatory drug in Advil, has the formula C13H18O2.
<IMAGE>
d. How many moles of ibuprofen contain 1.22 × 1023 atoms of C?
Balance each of the following by adding coefficients, and identify the type of reaction for each:
a.