Using the answer from problem 8.61, how many grams of nitrogen are in Whitney's lungs at STP if air contains 78% nitrogen?

Indicate which diagram (1, 2, or 3) represents the volume of the gas sample in a flexible container when each of the following changes (a to d) takes place:
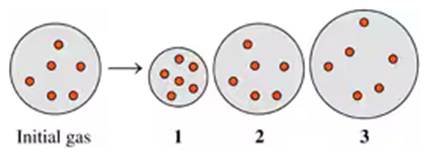
c. Atmospheric pressure decreases if temperature does not change.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Gas Laws

Flexible Container

Pressure-Volume Relationship
Two flasks of equal volume and at the same temperature contain different gases. One flask contains 5.0 g of O2 and the other flask contains 5.0 g of H2. Is each of the following statements true or false? Explain.
b. The pressures in the flasks are the same.
At 100 °C, which of the following diagrams (1, 2, or 3) represents a gas sample that exerts the:
b. highest pressure?
Indicate which diagram (1, 2, or 3) represents the volume of the gas sample in a flexible container when each of the following changes (a to d) takes place:
d. Doubling the atmospheric pressure and doubling the Kelvin temperature.
A balloon is filled with helium gas with a partial pressure of 1.00 atm and neon gas with a partial pressure of 0.50 atm. For each of the following changes (a to e) of the initial balloon, select the diagram (A, B, or C) that shows the final volume of the balloon:
<IMAGE>
c. All of the neon gas is removed (T and P do not change).
A balloon is filled with helium gas with a partial pressure of 1.00 atm and neon gas with a partial pressure of 0.50 atm. For each of the following changes (a to e) of the initial balloon, select the diagram (A, B, or C) that shows the final volume of the balloon:
<IMAGE>
d. The Kelvin temperature doubles and half of the gas atoms leak out (P does not change).
