Textbook Question
Are the following solutions isotonic, hypotonic, or hypertonic compared with a red blood cell?
c. 0.9% (m/v) NaCl
1577
views

 Verified step by step guidance
Verified step by step guidance

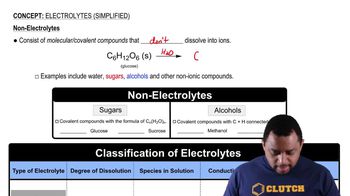

Are the following solutions isotonic, hypotonic, or hypertonic compared with a red blood cell?
c. 0.9% (m/v) NaCl
Match the diagrams with the following:
b. a nonpolar solute and a polar solvent
<IMAGE>
If all the solute is dissolved in diagram 1, how would heating or cooling the solution cause each of the following changes?
a. 2 to 3
<IMAGE>
Select the diagram that represents the solution formed by a solute <IMAGE> that is a
c. strong electrolyte
<IMAGE>
Select the container that represents the dilution of a 4% (m/v) KCl solution to give each of the following:
a. a 2% (m/v) KCl solution
<IMAGE>
A pickle is made by soaking a cucumber in brine, a salt-water solution. What makes the smooth cucumber become wrinkled like a prune?