Seeing common misconceptions using real-time questions

Melissa Hines
Professor of Chemistry, Cornell University
I used Learning Catalytics with my second semester General Chemistry course in the spring with 900 students. I decided to try Learning Catalytics since I hate "multiple-guess questions" that students can solve through a process of elimination. I was also intrigued by the possibility of graphical responses. Using open-ended questions in class allows me to challenge my students and understand their misconceptions.
Learning Catalytics gives me a deeper understanding of my students' conceptual misunderstandings. It compiles the answers in real-time and points out common misconceptions that are often small and surprising. Students sometimes get the "big picture," but stumble on a small detail that I find obvious. Who would have predicted that students could understand the Nernst equation without realizing that concentration increases when a solution evaporates? Learning Catalytics helps me catch these issues and respond to them in class. This feedback also helps me ask better, more conceptual questions.
I particularly like the region question format to zoom in on a graphic hot spot as well as the composite sketch question format since it shows me (and the students) all student inputs. Students like the graph help and I like how I was often surprised by a few wrong answers that I wouldn't have known to explain beforehand.
Region questions allow me to ask about changes in two variables at once — such as pH and pOH or ΔH and ΔS — while also presenting all possible answers to the students. This enables questions that probe multiple levels of understanding. As in the example shown, to get the pH/pOH question correct, students must first understand the effect of temperature on equilibrium (LeChâtelier's principle) and then they must understand that an increase in concentration leads to a decreaseon a p-scale (a tricky concept!). Finally, they can't get tripped by the common relation pH + pOH = 14, as that only applies at 25°C.
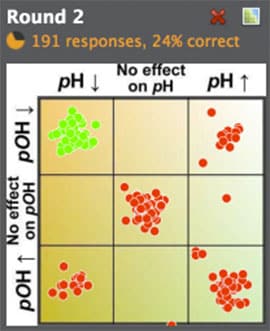
Interestingly, students prefer questions with a "trick" that fools a significant portion of the class, as these questions often highlight common errors on exams. Students were much less enthusiastic about questions that 80% of the class answered correctly.
At the end of the semester, I conducted a student survey and received over 500 responses. 75% of the students were satisfied or very satisfied with Learning Catalytics. Students especially liked questions that required a graphical response, and they noticed and appreciated that the questions were conceptual, not algorithmic. Many students noted that they thought they understood the topic during lecture until a Learning Catalytics question made them realize they didn't understand the material after all. Students also appreciated the ability to review the questions after class, especially in preparation for our exams. Additionally, students preferred Learning Catalytics over the standard campus-wide clicker system by a ratio of 2.7 to 1.
To enable the participation of students who didn't own or didn't bring a wifi-enabled device to class, I bought 5 Kindle Fires that could be borrowed from the podium. This covered my students' needs. When I teach General Chemistry next year, I look forward to using Learning Catalytics again.