Textbook Question
Some claim that the pH of your blood can be affected by eating acidic foods such as citrus. Do you believe this to be true? Explain your answer. (Hint: What happens when extra hydrogen ions are added to the blood?)
662
views

 Verified step by step guidance
Verified step by step guidance
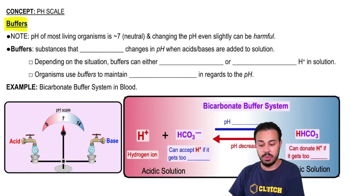

Some claim that the pH of your blood can be affected by eating acidic foods such as citrus. Do you believe this to be true? Explain your answer. (Hint: What happens when extra hydrogen ions are added to the blood?)
In certain types of radioactive decay, the isotope releases a particle called an alpha particle, which contains two protons and two neutrons. When this happens, is the product still the same element? Why or why not?
Considering that water is a main component of the juices in the stomach and intestines, explain why digestion of lipids is more complicated than digestion of carbohydrates and proteins.
Explain why monosaccharides are polar and fatty acids are nonpolar even though they both contain the same atoms.