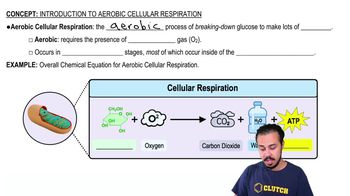
The final electron acceptor of the electron transport chain that functions in aerobic oxidative phosphorylation is
a. Oxygen.
b. Water.
c. NAD+.
d. Pyruvate.

 Verified step by step guidance
Verified step by step guidance


The final electron acceptor of the electron transport chain that functions in aerobic oxidative phosphorylation is
a. Oxygen.
b. Water.
c. NAD+.
d. Pyruvate.
In mitochondria, exergonic redox reactions
a. Are the source of energy driving prokaryotic ATP synthesis.
b. Provide the energy that establishes the proton gradient.
c. Reduce carbon atoms to carbon dioxide.
d. Are coupled via phosphorylated intermediates to endergonic processes.
What is the oxidizing agent in the following reaction?
Pyruvate + NADH + H+ → Lactate + NAD+
a. Oxygen
b. NADH
c. Lactate
d. Pyruvate
Most CO2 from catabolism is released during
a. Glycolysis.
b. The citric acid cycle.
c. Lactate fermentation.
d. Electron transport.
Step 3 in Figure 9.8 is a major point of regulation of glycolysis. The enzyme phosphofructokinase is allosterically regulated by ATP and related molecules (see Concept 8.5). Considering the overall result of glycolysis, would you expect ATP to inhibit or stimulate activity of this enzyme? Explain.
(Hint: Make sure you consider the role of ATP as an allosteric regulator, not as a substrate of the enzyme.)
The proton pump shown in Figures 7.17 and 7.18 is a type of ATP synthase (see Figure 9.14). Compare the processes shown in the two figures, and say whether they are involved in active or passive transport (see Concepts 7.3 and 7.4).