Textbook Question
Describe the formation of an aqueous KI solution, when solid KI dissolves in water.
1807
views

 Verified step by step guidance
Verified step by step guidance
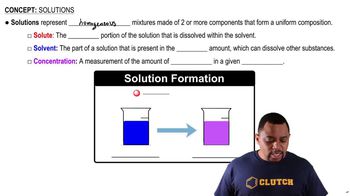

Describe the formation of an aqueous KI solution, when solid KI dissolves in water.
Water is a polar solvent and carbon tetrachloride (CCl4) is a nonpolar solvent. In which solvent is each of the following, which is found or used in the body, more likely to be soluble?
d. cholesterol (lipid), nonpolar
KF is a strong electrolyte, and HF is a weak electrolyte. How is the solution of KF different from that of HF?