Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
a.

 Verified step by step guidance
Verified step by step guidance


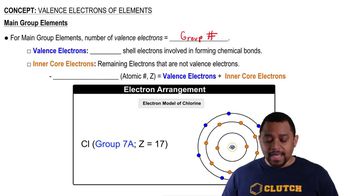
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
a.
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
b. NH3(g) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Calculate the number of equivalents in each of the following:
d. 3 moles of CO32–
An intravenous saline solution contains 154 mEq/L each of Na+ and Cl–. How many moles each of Na+ and Cl– are in 1.00 L of the saline solution?
An intravenous solution contains 40. mEq/L of Cl– and 15 mEq/L of HPO42–. If Na+ is the only cation in the solution, what is the Na+ concentration, in milliequivalents per liter?
When Michelle's blood was tested, the chloride level was 0.45 g/dL.
b. According to TABLE 9.6, is this value above, below, or within the normal range?