Textbook Question
Indicate whether each of the following statements is characteristic of an acid, a base, or both
b. neutralizes bases
1020
views

 Verified step by step guidance
Verified step by step guidance
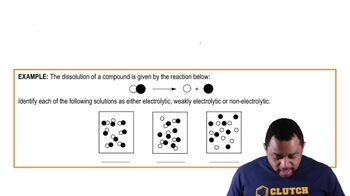
Indicate whether each of the following statements is characteristic of an acid, a base, or both
b. neutralizes bases
Indicate whether each of the following statements is characteristic of an acid, a base, or both
c. produces H⁺ ions in water
Indicate whether each of the following statements is characteristic of an acid, a base, or both
d. is named barium hydroxide
Indicate whether each of the following statements is characteristic of an acid, a base, or both:
a. neutralizes acids
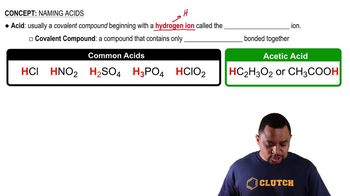
Write formulas for each of the following acids and bases:
f. hypochlorous acid
Identify the reactant that is a Brønsted–Lowry acid and the reactant that is a Brønsted–Lowry base in each of the following:
a. HI(aq) + H2O(l) → I-(aq) + H3O+(aq)