Textbook Question
Draw the condensed structural formula, or line-angle formula, if cyclic, for each of the following:
b. 1-bromo-3-hexyne
738
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 32b
Problem 32b Verified step by step guidance
Verified step by step guidance

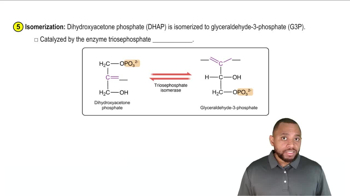

Draw the condensed structural formula, or line-angle formula, if cyclic, for each of the following:
b. 1-bromo-3-hexyne
Draw the condensed structural formula for each of the following:
a. trans-1-bromo-2-chloroethene
Draw the condensed structural formula for each of the following:
a. cis-1,2-difluoroethene
Draw the structural formula for the product in each of the following reactions:
b.
Draw the structural formula for the product in each of the following reactions:
a.