Textbook Question
Draw the condensed structural formulas for all the possible haloalkane isomers that have four carbon atoms and a bromine.
835
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 73b
Problem 73b Verified step by step guidance
Verified step by step guidance

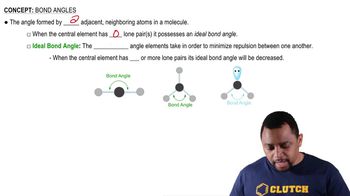
Draw the condensed structural formulas for all the possible haloalkane isomers that have four carbon atoms and a bromine.
Consider the compound ethylcyclopentane.
a. Draw the line-angle formula for ethylcyclopentane.
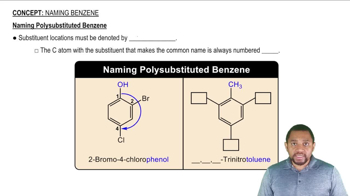
Explosives used in mining contain TNT, or trinitrotoluene.
<IMAGE>
a. If the functional group nitro is ―NO2 draw the line-angle formula for 2,4,6-trinitrotoluene, one isomer of TNT.