Textbook Question
Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°):
d.
834
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 15c
Problem 15c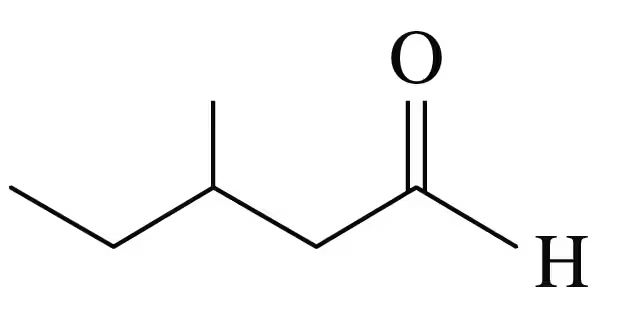
 Verified step by step guidance
Verified step by step guidance

Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°):
d.
Are each of the following soluble, slightly soluble, or insoluble in water? Explain.
a. CH3—CH2—CH2—OH
Give an explanation for each of the following observations:
b. 1-Propanol is soluble in water, but ethyl methyl ether is only slightly soluble.
Identify each of the following compounds as an aldehyde or a ketone:
d.
Identify each of the following compounds as an aldehyde or a ketone:
a.
Identify each of the following compounds as an aldehyde or a ketone:
d.