Textbook Question
Are each of the following soluble, slightly soluble, or insoluble in water? Explain.
a. CH3—CH2—CH2—OH
1580
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 15d
Problem 15d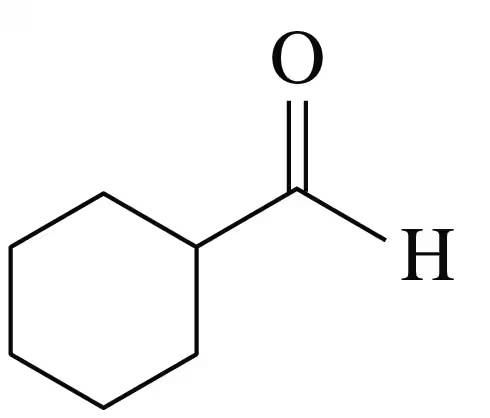
 Verified step by step guidance
Verified step by step guidance
Are each of the following soluble, slightly soluble, or insoluble in water? Explain.
a. CH3—CH2—CH2—OH
Give an explanation for each of the following observations:
b. 1-Propanol is soluble in water, but ethyl methyl ether is only slightly soluble.
Identify each of the following compounds as an aldehyde or a ketone:
c.
Identify each of the following compounds as an aldehyde or a ketone:
a.
Identify each of the following compounds as an aldehyde or a ketone:
d.
Give the common name for each of the following:
a.