What type of interaction would you expect between the R groups of the following amino acids in a quaternary structure?
d. alanine and proline

 Verified step by step guidance
Verified step by step guidance

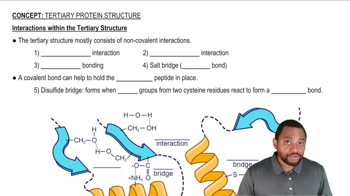

What type of interaction would you expect between the R groups of the following amino acids in a quaternary structure?
d. alanine and proline
A portion of a polypeptide chain contains the following sequence of amino acids:
—Leu—Val—Cys—Asp—
a. Which amino acids are likely to be found on the inside of the protein structure? Why?
A portion of a polypeptide chain contains the following sequence of amino acids:
—Leu—Val—Cys—Asp—
c. How does the primary structure of a protein affect its tertiary structure?
In myoglobin, about one-half of the 153 amino acids have nonpolar R groups.
b. Where would you expect the polar R groups to be in the tertiary structure?
Indicate whether each of the following statements describes primary, secondary, tertiary, or quaternary protein structure:
c. Several polypeptides in a beta-pleated sheet are held together by hydrogen bonds between adjacent chains.
Indicate whether each of the following statements describes primary, secondary, tertiary, or quaternary protein structure:
b. Protein chains of collagen form a triple helix.