Textbook Question
Refer to the diagram of the citric acid cycle in Figure 18.13 to answer each of the following:
d. What are the decarboxylation reactions?
486
views

 Verified step by step guidance
Verified step by step guidance
Refer to the diagram of the citric acid cycle in Figure 18.13 to answer each of the following:
d. What are the decarboxylation reactions?
Refer to the diagram of the citric acid cycle in Figure 18.13 to answer each of the following:
b. What are the four-carbon compounds?
Refer to the diagram of the citric acid cycle in Figure 18.13 to answer each of the following:
d. In which reactions are secondary alcohols oxidized?
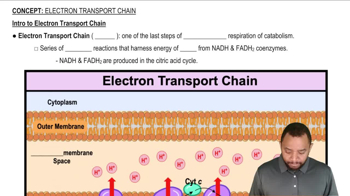
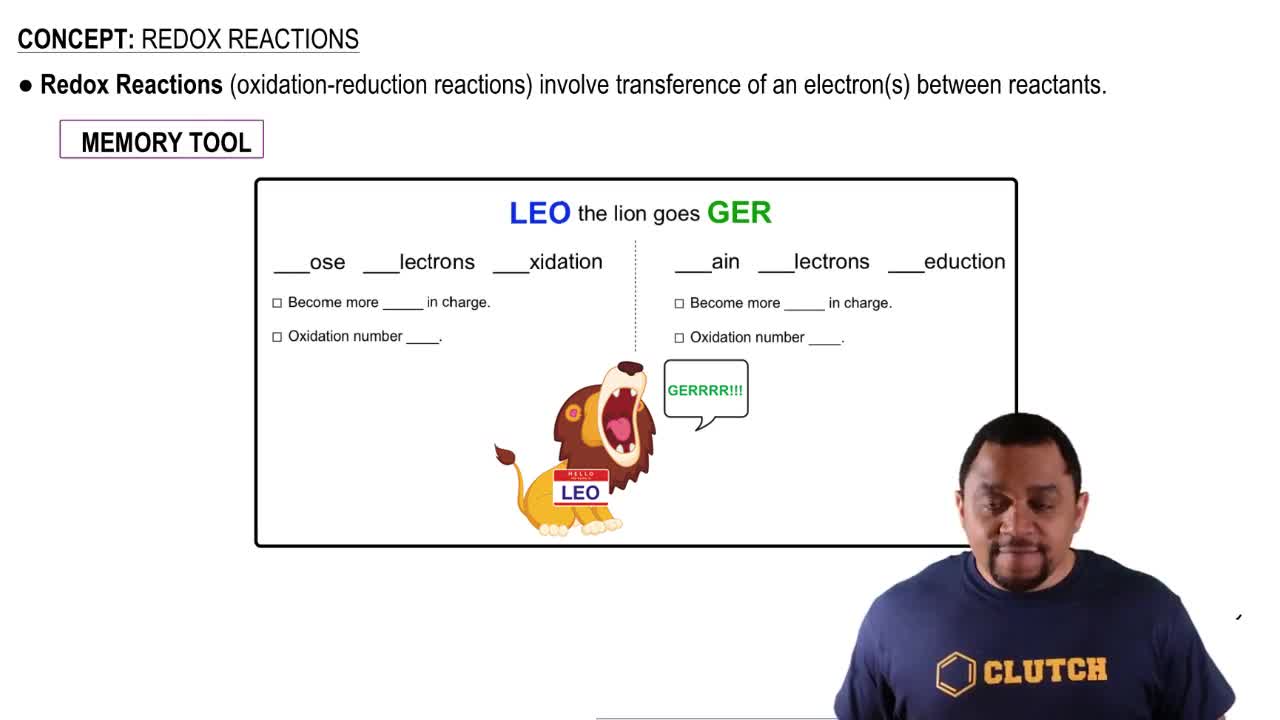
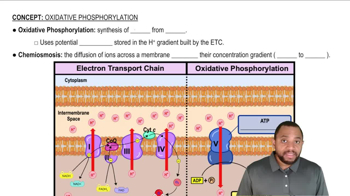
How is NADH oxidized in electron transport?
How is the H+ gradient established?
How are glycolysis and the citric acid cycle linked to the production of ATP by electron transport?