What is the density (g/mL) of each of the following samples?
b. A syrup is added to an empty container with a mass of 115.25 g. When 0.100 pt of syrup is added, the total mass of the container and syrup is 182.48 g.
<IMAGE>

 Verified step by step guidance
Verified step by step guidance



What is the density (g/mL) of each of the following samples?
b. A syrup is added to an empty container with a mass of 115.25 g. When 0.100 pt of syrup is added, the total mass of the container and syrup is 182.48 g.
<IMAGE>
What is the density (g/mL) of each of the following samples?
a. An ebony carving has a mass of 275 g and a volume of 207 cm3.
What is the density (g/mL) of each of the following samples?
b. A 14.3 - cm3 sample of tin has a mass of 0.104 kg.
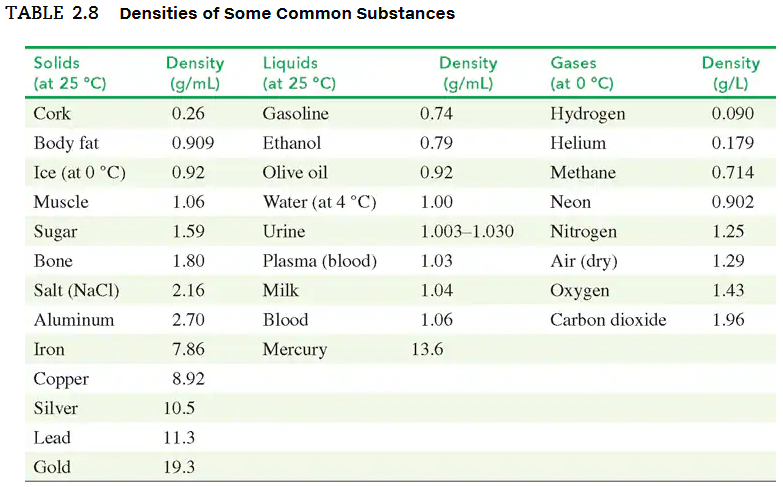
In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. You take it to a lab, where you find it has a mass of 217 g and a volume of 19.2 cm3. Using TABLE 2.8, what is the metal you found?
Solve each of the following problems:
a. A urine sample has a density of 1.030 g/mL. What is the specific gravity of the sample?
Solve each of the following problems:
c. The specific gravity of a vegetable oil is 0.92. What is the mass, in grams, of 750 mL of vegetable oil?