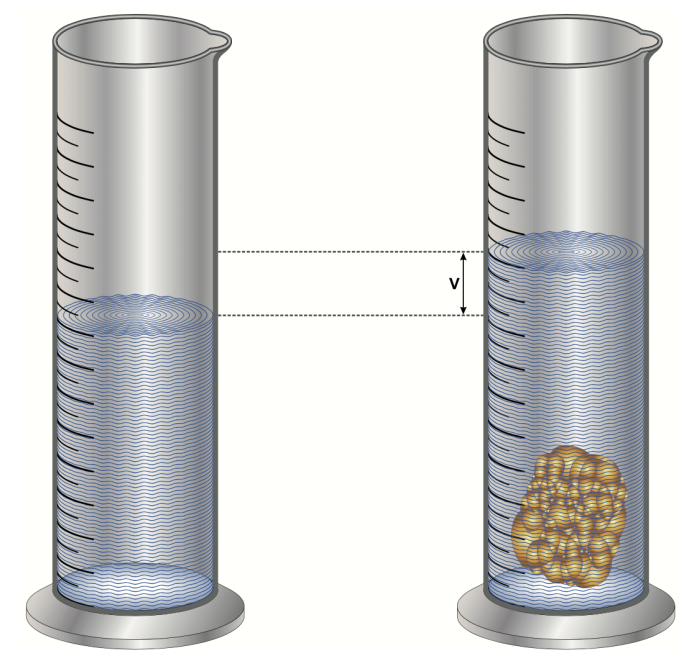
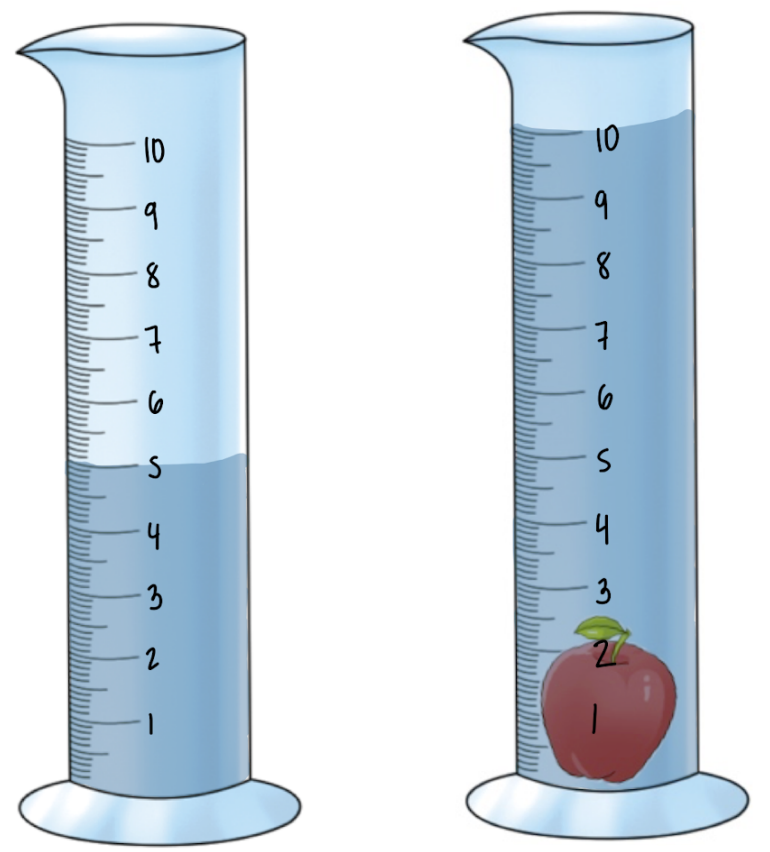
To calculate the density of non-geometric objects, the method of water displacement is employed. Water displacement refers to the volume of water that is moved aside when an object is fully submerged in water. This technique is particularly useful for determining the volume of irregularly shaped objects.
For instance, consider a scenario where we need to find the volume of a non-geometric object submerged in water. Initially, we measure the volume of water in a container, which is 3.5 milliliters. After placing the object in the water, the new water level rises to approximately 5.8 milliliters. To find the volume of the object, we subtract the initial water volume from the final water volume:
Volume of the object = Final volume - Initial volume = 5.8 \, \text{ml} - 3.5 \, \text{ml} = 2.3 \, \text{ml}.
Thus, the volume of the non-geometric object is 2.3 milliliters. This method not only provides a straightforward way to measure volume but also lays the groundwork for further calculations, such as determining density using the formula:
\[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} \]
In this context, knowing the mass of the object allows for the calculation of its density, which is a crucial property in various scientific applications.