Textbook Question
Give the symbol of the element described by each of the following:
a. the alkaline earth metal in Period 2
1417
views

 Verified step by step guidance
Verified step by step guidance
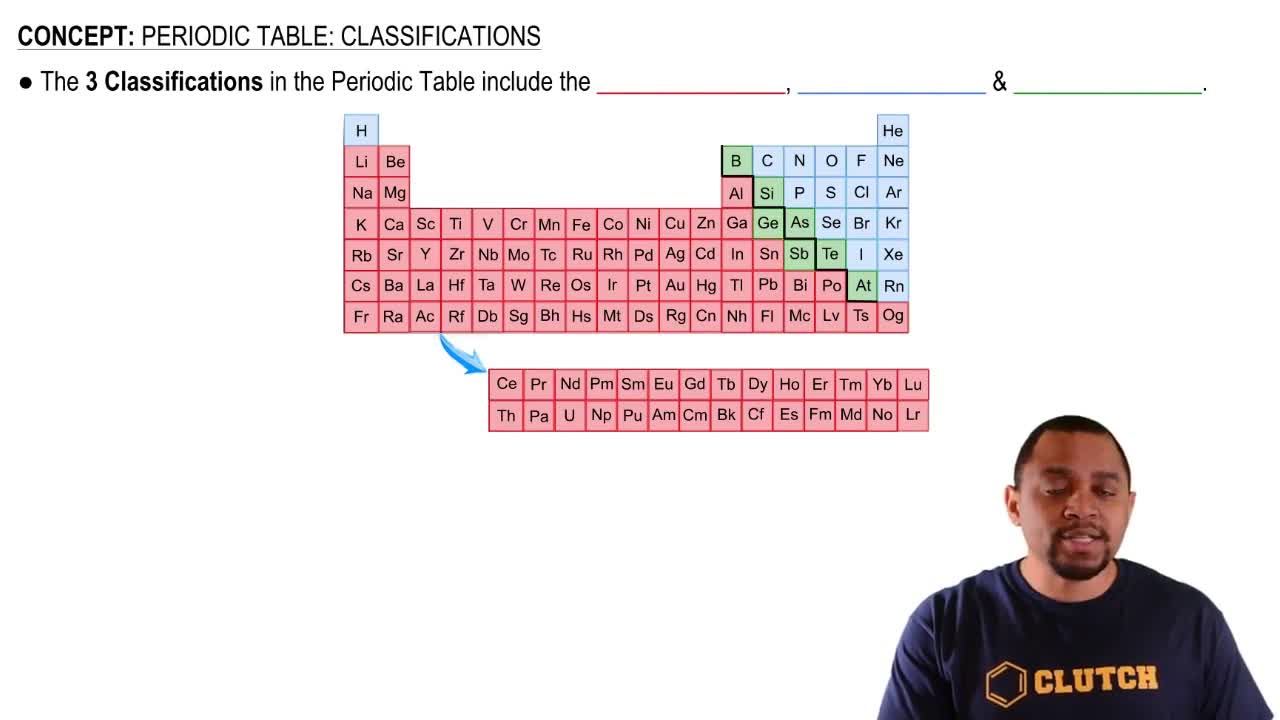


Give the symbol of the element described by each of the following:
a. the alkaline earth metal in Period 2
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
e. located in Group 8A (18)
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
a. located in Group 2A (2)
Using the Chemistry Link to Health: Elements Essential to Health, answer each of the following:
a. What is a macromineral?
Using the Chemistry Link to Health: Elements Essential to Health, answer each of the following:
b. What is the role of sulfur in the human body?
Using the Chemistry Link to Health: Elements Essential to Health, answer each of the following:
c. How many grams of sulfur would be a typical amount in a 60.-kg adult?