The dosage of technetium-99m for a lung scan is 20. µCi/kg of body mass. How many millicuries of technetium-99m should be given to a 50.0-kg person (1 mCi = 1000 µCi)

Strontium-85, used for bone scans, has a half-life of 65 days.
b. How long will it take for the radiation level of strontium-85 to drop to one-eighth of its original level?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Half-Life
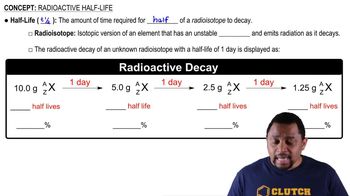
Exponential Decay

Fractional Reduction
Suppose a person absorbed 50 mrad of alpha radiation. What would be the equivalent dose in millisieverts?
For each of the following, indicate if the number of half-lives elapsed is:
1. one half-life
2. two half-lives
3. three half-lives
c. a sample of Au-198 with a half-life of 2.7 days after 5.4 days
Fluorine-18, which has a half-life of 110 min, is used in PET scans.
b. If 100. mg of fluorine-18 is shipped at 8:00 a.m., how many milligrams of the radioisotope are still active when the sample arrives at the radiology laboratory at 1:30 p.m.?
Bone and bony structures contain calcium and phosphorus.
a. Why would the radioisotopes calcium-47 and phosphorus-32 be used in the diagnosis and treatment of bone diseases?
Bone and bony structures contain calcium and phosphorus.
b. During nuclear tests, scientists were concerned that strontium-85, a radioactive product, would be harmful to the growth of bone in children. Explain.
