Textbook Question
One of the ions of tin is tin(IV).
b. How many protons and electrons are in the ion?
1738
views

 Verified step by step guidance
Verified step by step guidance


One of the ions of tin is tin(IV).
b. How many protons and electrons are in the ion?
Draw the Lewis structure for each of the following:
b. H2NOH (N is the central atom)
Draw the Lewis structure for each of the following:
a. H3COCH3 (the atoms are in the order C O C)
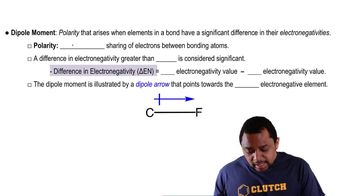
Show the dipole arrow for each of the following bonds:
a. Si―Cl
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
a. Si and Cl
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
b. C and C