Textbook Question
Draw the Lewis structure for each of the following:
b. H2NOH (N is the central atom)
1552
views

 Verified step by step guidance
Verified step by step guidance
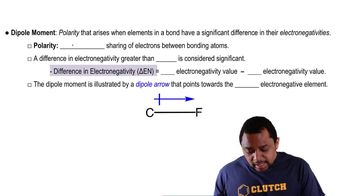


Draw the Lewis structure for each of the following:
b. H2NOH (N is the central atom)
Draw the Lewis structure for each of the following:
a. H3COCH3 (the atoms are in the order C O C)
Select the more polar bond in each of the following pairs:
c. Br―Cl or S―Cl
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
a. Si and Cl
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
b. C and C
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
c. Na and Cl