Using the models of the molecules (black = C, white = H, yellow = S, red = O), determine each of the following for models of compounds 1 and 2:
c. number of moles in 10.0 g


 Verified step by step guidance
Verified step by step guidance


Using the models of the molecules (black = C, white = H, yellow = S, red = O), determine each of the following for models of compounds 1 and 2:
c. number of moles in 10.0 g
Ibuprofen, an anti-inflammatory drug in Advil, has the formula C13H18O2.
<IMAGE>
d. How many moles of ibuprofen contain 1.22 × 1023 atoms of C?
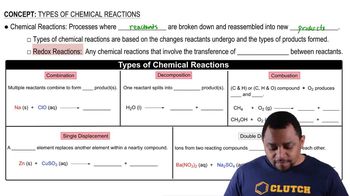
Balance each of the following by adding coefficients, and identify the type of reaction for each:
a.
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
a. write the formula for each of the reactants and products.
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
b. write a balanced equation for the reaction.
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
c. indicate the type of reaction as combination, decomposition, single replacement, double replacement, or combustion.