Textbook Question
What is the difference between a 5.00% (m/m) glucose solution and a 5.00% (m/v) glucose solution?
2173
views

 Verified step by step guidance
Verified step by step guidance


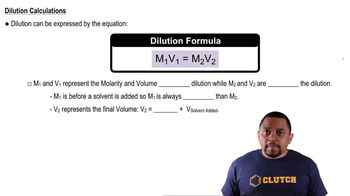
What is the difference between a 5.00% (m/m) glucose solution and a 5.00% (m/v) glucose solution?
What is the difference between a 10.0% (v/v) methanol (CH4O) solution and a 10.0% (m/m) methanol solution?
Calculate the mass/volume percent (m/v) for the solute in each of the following:
a. 75 g of Na2SO4 in 250 mL of Na2SO4 solution
A patient receives 100. mL of 20.% (m/v) mannitol solution every hour.
b. How many grams of mannitol does the patient receive in 12 h?
A patient needs 100. g of glucose in the next 12 h. How many liters of a 5% (m/v) glucose solution must be given?
Determine the final volume, in milliliters, of each of the following:
b. a 2.0% (m/v) LiCl solution prepared from 50.0 mL of a 10.0% (m/v) LiCl solution