You need 500. mL of a 5.0% (m/v) glucose solution. If you have a 25% (m/v) glucose solution on hand, how many milliliters do you need?
Ch.9 Solutions

Timberlake13th EditionChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9780134421353Not the one you use?Change textbook
Chapter 9, Problem 67
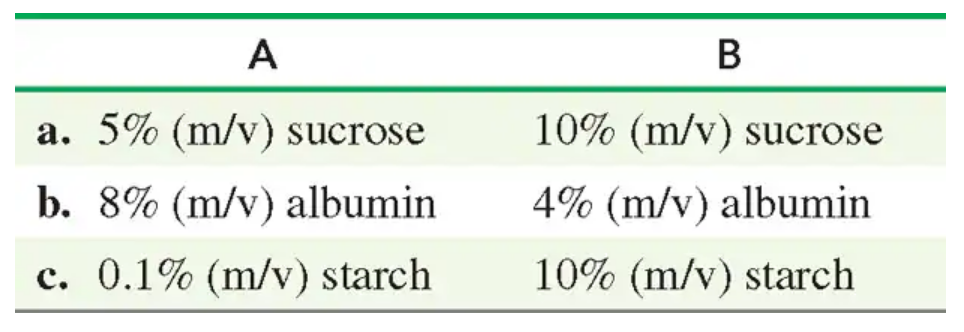
Indicate the compartment (A or B) that will increase in volume for each of the following pairs of solutions separated by a semipermeable membrane:
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of osmosis. Osmosis is the movement of water across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration.
Step 2: Analyze the solutions provided. Compartment I contains a 3% (m/v) solution of MgCl₂, while Compartment II contains a 6% (m/v) solution of MgCl₂. The semipermeable membrane allows water to pass but not solutes.
Step 3: Determine the direction of water movement. Water will move from the compartment with lower solute concentration (Compartment I) to the compartment with higher solute concentration (Compartment II) to equalize the concentrations.
Step 4: Predict the volume change. As water moves into Compartment II, its volume will increase, while the volume of Compartment I will decrease.
Step 5: Conclude that Compartment II will increase in volume due to the osmotic movement of water from Compartment I to Compartment II.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Osmosis
Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. This process continues until equilibrium is reached, meaning the concentrations on both sides of the membrane become equal. Understanding osmosis is crucial for predicting which compartment will increase in volume when two solutions with different concentrations are separated by a membrane.
Recommended video:
Guided course

Osmosis Example 1
Solute Concentration
Solute concentration refers to the amount of solute present in a given volume of solution. In the context of the question, the 3% (m/v) MgCl2 solution has a lower solute concentration compared to the 6% (m/v) MgCl2 solution. The difference in solute concentration drives the osmotic movement of water, leading to volume changes in the compartments.
Recommended video:
Guided course

Percent Concentrations Concept 1
Tonicity
Tonicity describes the relative concentration of solutes in two solutions separated by a semipermeable membrane, influencing the direction of water movement. A solution can be isotonic, hypertonic, or hypotonic relative to another. In this case, the 3% MgCl2 solution is hypotonic compared to the 6% MgCl2 solution, meaning water will move from the 3% solution to the 6% solution, resulting in an increase in volume in the latter.
Recommended video:
Guided course
Osmosis Example 2
Related Practice
Textbook Question
2203
views
Textbook Question
Identify each of the following as characteristic of a solution, colloid, or suspension:
a. a mixture that cannot be separated by a semipermeable membrane
2172
views
Textbook Question
Identify each of the following as characteristic of a solution, colloid, or suspension:
a. particles of this mixture remain inside a semipermeable membrane but pass through filters
1176
views
Textbook Question
Are the following solutions isotonic, hypotonic, or hypertonic compared with a red blood cell?
c. 0.9% (m/v) NaCl
1577
views
Textbook Question
Match the diagrams with the following:
b. a nonpolar solute and a polar solvent
<IMAGE>
1451
views
Textbook Question
If all the solute is dissolved in diagram 1, how would heating or cooling the solution cause each of the following changes?
a. 2 to 3
<IMAGE>
970
views
