Textbook Question
The use of a proton motive force to generate ATP is __________.
661
views

 Verified step by step guidance
Verified step by step guidance
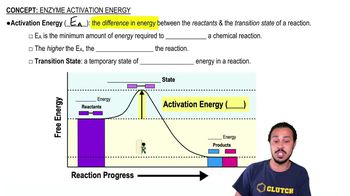

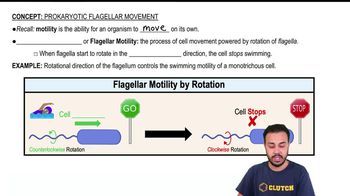
The use of a proton motive force to generate ATP is __________.
Why do we breathe oxygen and give off carbon dioxide?
Microbes that reduce N2 to NH3 engage in nitrogen ___________ .
Why do cyanobacteria and algae take in carbon dioxide and give off oxygen?
Coenzymes are _______.
a. Types of apoenzymes
b. Proteins
c. Inorganic cofactors
d. Organic cofactors
What happens to the carbon atoms in sugar catabolized by Escherichia coli?