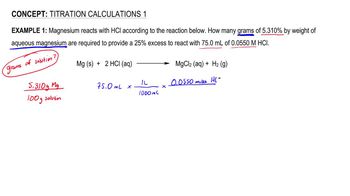
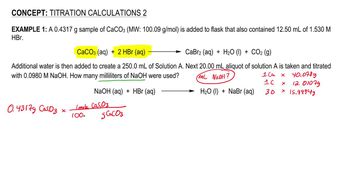
1. Chemical Measurements
Volumetric Titrations
1. Chemical Measurements
Volumetric Titrations
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
A 1.000 g sample of Na2CO3 (MW: 105.99 g/mol) is dissolved in enough water to make 200.0 mL of solution. A 25.00 mL aliquot required 32.18 mL of HCl to completely neutralize it. What is the molar concentration of HCl?
Na2CO3 (aq) + 2 HCl (aq) → 2 KCl (aq) + H2O(l) + CO2 (g)
311views2rank - Multiple ChoiceIn volumetric titrations, standardization is the process of titrating a solution prepared from which of the following?8views
- Multiple ChoiceIn a volumetric titration, what is the analyte?6views
- Multiple ChoiceIn volumetric titrations, what is the primary purpose of standardization of a titrant solution?6views