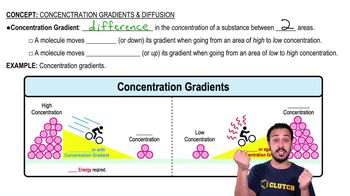
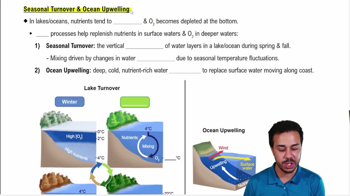
When H2 and CO2 react, acetic acid can be formed spontaneously while the production of formaldehyde requires an input of energy. Which of these conclusions can be drawn from this observation?
a. More heat is released when formaldehyde is produced compared to the production of acetic acid.
b. Compared to the reactants that it is formed from, formaldehyde has more potential energy than does acetic acid.
c. Entropy decreases when acetic acid is produced and increases when formaldehyde is produced.
d. Only acetic acid could be produced under conditions that existed in early Earth.