Gibbs free energy is a crucial concept in understanding chemical reactions and their spontaneity, particularly in the context of cell biology. It serves as a measure to determine whether a reaction can occur without external assistance, indicating that spontaneous reactions are thermodynamically favorable. This means they can happen naturally, often resulting in an increase in entropy, or disorder, within the universe. The Gibbs free energy at a specific moment in a reaction is denoted as \( G \), while the change in Gibbs free energy during a reaction is represented as \( \Delta G \). This value is dynamic and varies as the reaction progresses towards equilibrium.
Equilibrium is defined as the state where the forward and reverse reactions occur at equal rates. However, it is important to note that biological systems do not exist at equilibrium; instead, they operate in a state where reactions are continuously striving for equilibrium without actually reaching it. This dynamic state is essential for life, as it allows for the ongoing biochemical processes necessary for cellular function.
In terms of calculations, \( \Delta G \) is used to assess the spontaneity of a reaction based on the concentrations of reactants and products at a given time. In contrast, the standard free energy change, denoted as \( \Delta G^\circ \), evaluates spontaneity under standard conditions (25 degrees Celsius and 1 atm pressure). This standardization is vital for comparing the thermodynamic favorability of different reactions, as it removes the variability introduced by differing concentrations.
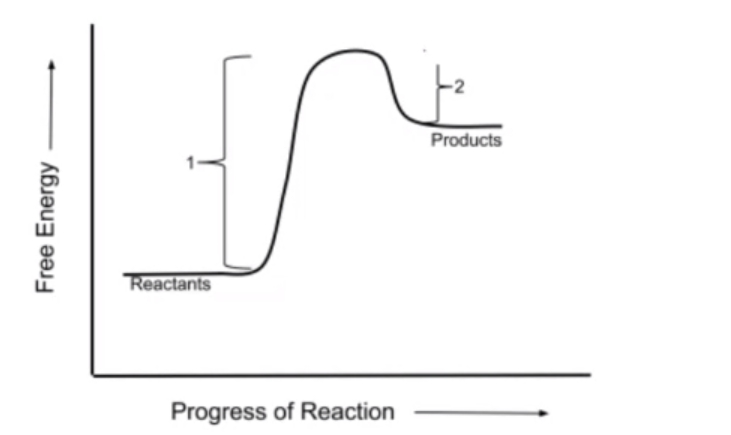
Additionally, reactions can be classified as exergonic or endergonic. Exergonic reactions release energy, making them more thermodynamically favorable, and are characterized by a negative \( \Delta G \) value, indicating that the free energy of the products is lower than that of the reactants. Conversely, endergonic reactions absorb energy, resulting in a positive \( \Delta G \) value, where the products have a higher free energy than the reactants. At equilibrium, \( \Delta G \) equals zero, signifying that no net change occurs in the concentrations of reactants and products.
Visual representations of these concepts often include graphs plotting free energy against the progress of the reaction. In exergonic reactions, the graph shows a decrease in free energy from reactants to products, while endergonic reactions display an increase. Understanding these principles is essential for grasping the biochemical processes that sustain life, as they highlight the interplay between energy changes and reaction dynamics.