Textbook Question
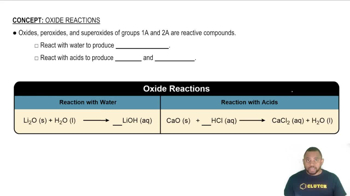
Because the oxide ion is basic, metal oxides react readily with acids. (a) Write the net ionic equation for the following reaction: FeO(s) + 2 HClO4(aq) → Fe(ClO4)2(aq) + H2O(l) (b) Based on the equation in part (a), write the net ionic equation for the reaction that occurs between NiO(s) and an aqueous solution of nitric acid.
508
views