Textbook Question
Arrange each of the following sets of atoms and ions, in orderof increasing size: (a) Pb, Pb2+, Pb4+
1053
views

 Verified step by step guidance
Verified step by step guidance

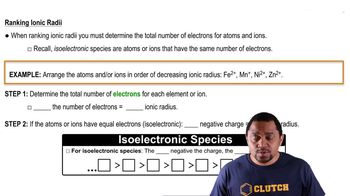

Provide a brief explanation for each of the following: (a) Cl- is larger than Ar. (b) P3- is larger than S2-. (c) K+ is larger than Na+. (d) F- is larger than F.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 201 pm (Li–F), 282 pm (Na–Cl), 330 pm (K–Br), and 367 pm (Rb–I), respectively. (b) Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.8.
Which element has the highest second ionization energy: Li, K, or Be?