Balance each of the following neutralization equations:
a. HNO3(aq) + Ba(OH)2(s) → H2O(l) + Ba(NO3)2(aq)

 Verified step by step guidance
Verified step by step guidance

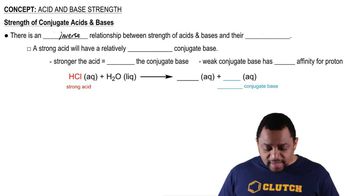

Balance each of the following neutralization equations:
a. HNO3(aq) + Ba(OH)2(s) → H2O(l) + Ba(NO3)2(aq)
Write a balanced equation for the neutralization of each of the following:
c. HNO3(aq) and Mg(OH)2(s)
If 32.8 mL of a 0.162 M NaOH solution is required to titrate 25.0 mL of a solution of H2SO4, what is the molarity of the H2SO4 solution?
H2SO4(aq) + 2 KOH(aq) → 2 H2O(l) + K2SO4(aq)
Someone with severe diabetes obtains energy by the breakdown of fats, which produce large amounts of acidic substances. How would this affect the pH of the blood plasma?
When food enters the stomach, HCl is released and the [H3O+] of the stomach fluid rises to 4 × 10–2 M. What is the pH of the stomach fluid?
Write the balanced chemical equation for the neutralization reaction of stomach acid HCl with Al(OH)3, an ingredient in some antacids.