Textbook Question
Identify each of the following as an acid, base, or salt, and give its name:
e. H2CO3
1295
views

 Verified step by step guidance
Verified step by step guidance

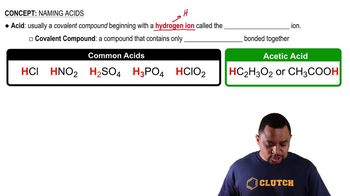

Identify each of the following as an acid, base, or salt, and give its name:
e. H2CO3
Identify each of the following as an acid, base, or salt, and give its name:
a. H3PO4
Determine the pH for the following solutions:
c. [H3O+] = 0.0001 M
Solution X has a pH of 9.0, and solution Y has a pH of 7.0.
a. Which solution is more acidic?
A 0.205 M NaOH solution is used to titrate 20.0 mL of a solution of H2SO4. If 45.6 mL of the NaOH solution is required to reach the endpoint, what is the molarity of the H2SO4 solution?
H2SO4(aq) + 2NaOH(aq) → 2H2O(l) + Na2SO4
Calculate the volume, in milliliters, of a 0.150 M NaOH solution that will completely neutralize each of the following:
a. 25.0 mL of a 0.288 M HCl solution