Textbook Question
Identify each of the following properties as more typical of an organic or inorganic compound:
c. contains covalent bonds
1080
views

 Verified step by step guidance
Verified step by step guidance


Identify each of the following properties as more typical of an organic or inorganic compound:
c. contains covalent bonds
Match each of the following physical and chemical properties with ethane, C2H6 or sodium bromide, NaBr:
a. boils at -89 °C
Match each of the following physical and chemical properties with ethane, C2H6 or sodium bromide, NaBr:
b. burns vigorously in air
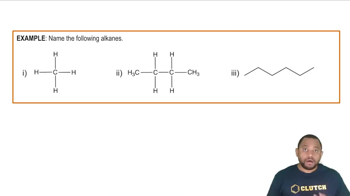
Give the IUPAC name for each of the following alkanes and cycloalkanes:
c.
Draw the condensed structural formula for alkanes or the line-angle formula for cycloalkanes for each of the following:
c. heptane
Indicate whether each of the following pairs represent structural isomers or the same molecule:
a.