Textbook Question
Classify each of the following proteins according to its function:
c. casein, milk protein
973
views

 Verified step by step guidance
Verified step by step guidance
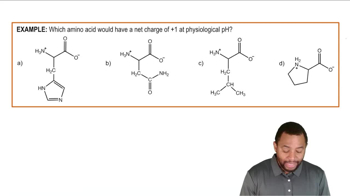


Classify each of the following proteins according to its function:
c. casein, milk protein
How does the polarity of the R group in leucine compare to the R group in serine?
Draw the structure for each of the following amino acids at physiological pH:
a. lysine
Give the name for the amino acid represented by each of the following abbreviations:
d. Cys
Give the name for the amino acid represented by each of the following abbreviations:
d. G
Draw the condensed structural formula for each of the following peptides, and give its three-letter and one-letter abbreviations:
a. alanylcysteine