Textbook Question
In the chemiosmotic model, how is energy provided to synthesize ATP?
1589
views

 Verified step by step guidance
Verified step by step guidance
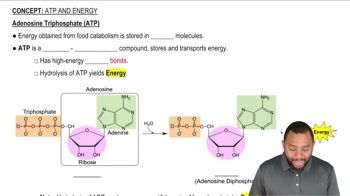


In the chemiosmotic model, how is energy provided to synthesize ATP?
What metabolic substrate(s) can be produced from the carbon atoms of each of the following amino acids?
a. histidine
What metabolic substrate(s) can be produced from the carbon atoms of each of the following amino acids?
d. phenylalanine
State if each of the following processes release or require ATP:
f. first six reactions of glycolysis
State if each of the following processes release or require ATP:
g. activation of a fatty acid
Match the following ATP yields to reactions a to g:
1.5 ATP 2.5 ATP 7 ATP 10 ATP
12 ATP 32 ATP 36 ATP
d. Acetyl CoA goes through one turn of the citric acid cycle.