Textbook Question
Describe each of the following as a physical or chemical property:
a. Chromium is a steel-gray solid.
1505
views

 Verified step by step guidance
Verified step by step guidance
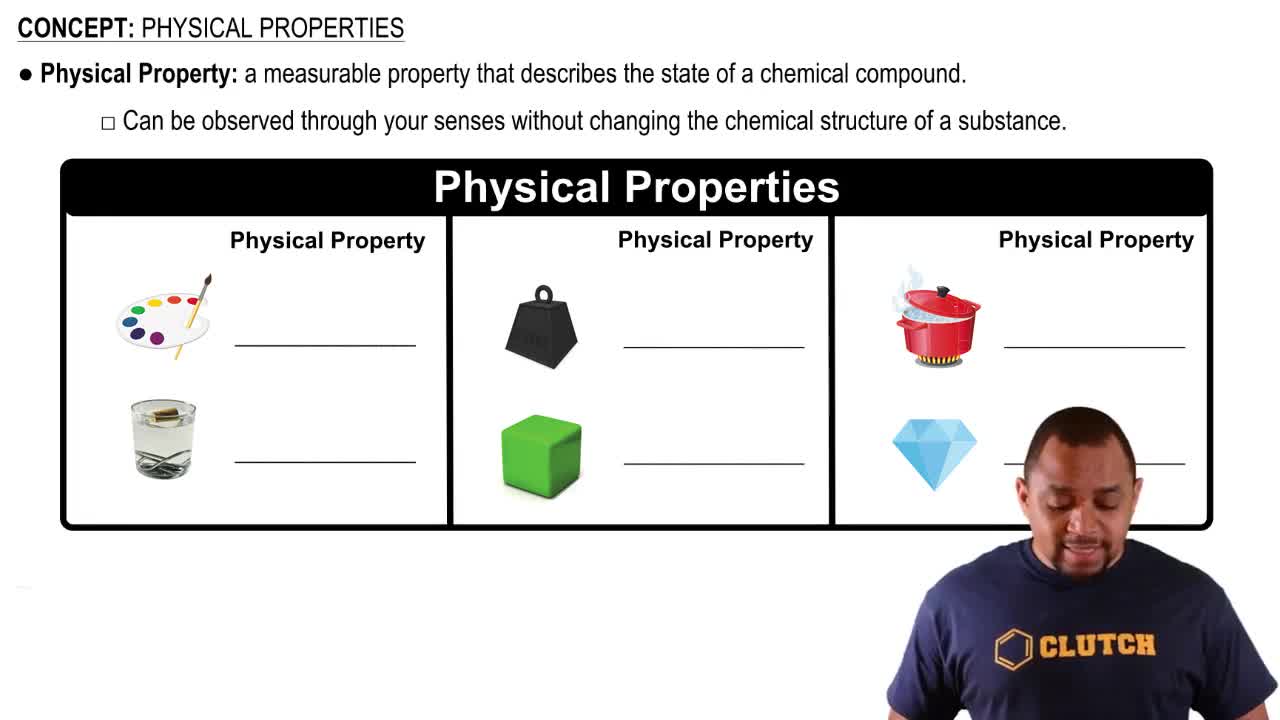


Describe each of the following as a physical or chemical property:
a. Chromium is a steel-gray solid.
Describe each of the following as a physical or chemical property:
b. Hydrogen reacts readily with oxygen.
Describe each of the following as a physical or chemical property:
a. Neon is a colorless gas at room temperature.
Describe each of the following as a physical or chemical property:
e. Propane gas is compressed to a liquid for placement in a small cylinder.
What type of change, physical or chemical, takes place in each of the following?
d. A puzzle is cut into 1000 pieces.
What type of change, physical or chemical, takes place in each of the following?
c. A tree is cut into boards at a saw mill.