Match the subatomic particles (1 to 3) to each of the descriptions below:
1. protons
2. neutrons
3. electrons
b. surround the nucleus

 Verified step by step guidance
Verified step by step guidance

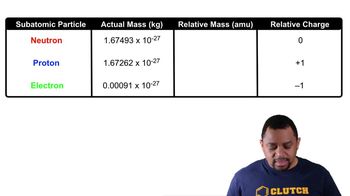

Match the subatomic particles (1 to 3) to each of the descriptions below:
1. protons
2. neutrons
3. electrons
b. surround the nucleus
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
a. What atoms have the same number of protons?
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
c. Which atoms have the same mass number?
Indicate if the atoms in each pair have the same number of protons, neutrons, or electrons.
c. 4018Ar, 3917Cl
Complete the following table for the three naturally occurring isotopes of silicon, the major component in computer chips:
For each representation of a nucleus A through E, write the atomic symbol and identify which are isotopes.
a. <IMAGE>