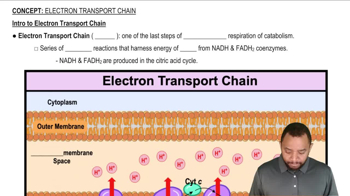
Complete each of the following nuclear equations:
d. 23m12Mg → ? + 00γ

 Verified step by step guidance
Verified step by step guidance

Complete each of the following nuclear equations:
d. 23m12Mg → ? + 00γ
Write the balanced nuclear equation for each of the following:
a. When two oxygen-16 atoms collide, one of the products is an alpha particle.
Write the balanced nuclear equation for each of the following:
a. Polonium-210 decays to give lead-206.
Where does fusion occur naturally?
All the elements beyond uranium, the transuranium elements, have been prepared by bombardment and are not naturally occurring elements. The first transuranium element neptunium, Np, was prepared by bombarding U-238 with neutrons to form a neptunium atom and a beta particle. Complete the following equation:
10n + 23892U →? + ?
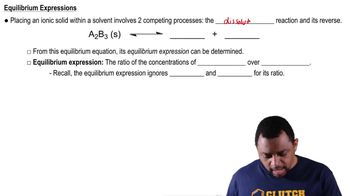
The half-life for the radioactive decay of Ce-141 is 32.5 days. If a sample has an activity of 4.0 µCi after 130 days have elapsed, what was the initial activity, in microcuries, of the sample?