Textbook Question
How does the octet rule explain the formation of a magnesium ion?
1402
views


 Verified step by step guidance
Verified step by step guidance
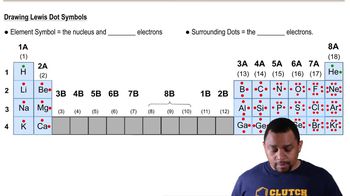


How does the octet rule explain the formation of a magnesium ion?
What noble gas has the same electron arrangement as the magnesium ion?
Why are Group 1A (1) and Group 2A (2) elements found in many compounds, but not Group 8A (18) elements?
Consider the following Lewis symbols for elements X and Y:
c. What ions would be formed by X and Y?
Consider the following Lewis symbols for elements X and Y:
d. What would be the formula of a compound of X and Y?
Consider the following Lewis symbols for elements X and Y:
e. What would be the formula of a compound of X and sulfur?