Name the following polyatomic ions:
c. HSO3-

 Verified step by step guidance
Verified step by step guidance


Name the following polyatomic ions:
c. HSO3-
Write the formula for the polyatomic ion and name each of the following compounds:
d. Fe(HCO3)3
Draw the Lewis structure for each of the following molecules:
d. ClNO2 (N is the central atom)
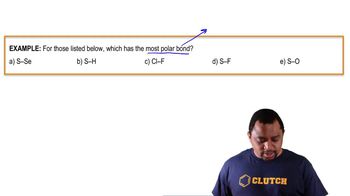
For each of the following bonds, indicate the positive end with 𝛿⁺ and the negative end with 𝛿⁻ . Draw an arrow to show the dipole for each.
a. N and F
Choose the shape (1 to 6) that matches each of the following descriptions (a to c):
1. linear
2. bent (109°)
3. trigonal planar
4. bent (120°)
5. trigonal pyramidal
6. tetrahedral
b. a molecule with a central atom that has four electron groups and three bonded atoms
Complete each of the following statements for a molecule of H2S:
c. The number of atoms attached to the central S atom is _______.